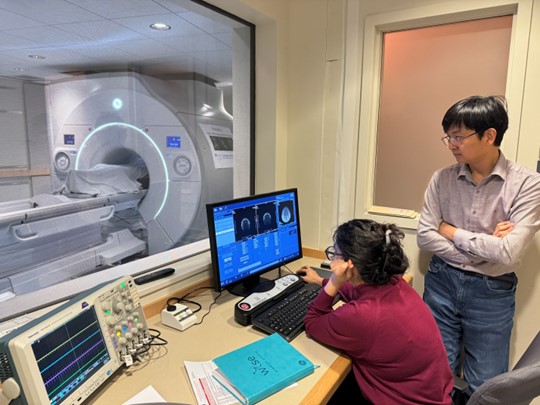
Brigham and Women’s Hospital has acquired a new high-performance 3T MRI scanner for human neuroimaging research, with support from the Massachusetts Life Sciences Center and Harvard Medical School. The new scanner, termed MAGNUS, was developed by GE under contract from the Department of Defense and is based on a head-only design that achieves very high gradient strengths. This is especially advantageous for diffusion imaging and for the study of brain microarchitecture, but it also provides many technical advantages for other forms of MRI. The MAGNUS is installed in the BWH MRI imaging center at 221 Longwood Avenue, and will be operated as a core facility, open to internal and external users.
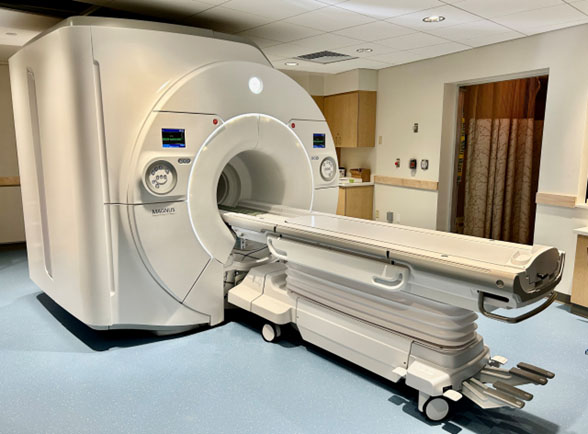
The MAGNUS (Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning) system was developed by GE under a contract from the US Department of Defense, with the goal of imaging the microstructure of the human brain at very high resolution. This system is based on a unique head-only insert that is installed in a standard clinical 3T magnet; the narrower bore at the head allows the MAGNUS to achieve exceptional gradient performance, measured by amplitude and slew rate. The MAGNUS achieves a maximal gradient performance of 300 mT/m and 750 T/m/s slew rate, compared to typical clinical whole-body MRI systems (80 mT/m and 200 T/m/s). The only comparable scanner in the Boston area is the ‘Connectom 2.0’ at MGH Charlestown (500 mT/m and 600 T/m/s).
The high gradient performance of the MAGNUS translates to a more than 4-fold improvement over typical 3T scanners, providing excellent resolution with shorter scan times and reduced distortion, and revealing details of brain microstructure that are undetectable on other 3T or even 7T scanners. We expect to achieve even higher performance in future, through further upgrades to the gradient driver electronics, along with a novel 64-channel head coil that will be developed by Nova Medical as part of this project.
The head-only design of the MAGNUS also minimizes the problem of cardiac and peripheral nerve stimulation, which has limited the performance of other advanced scanners. The MAGNUS design has additional advantages, including a wide bore, enabling scans of large or claustrophobic subjects who often cannot tolerate the narrow bore of a typical 3T scanner or an even narrower 7T scanner.
The MAGNUS was developed specifically for neuroimaging research, and we anticipate that it will enable our users to take advantage of many new imaging methods that are currently in development. In addition to neuroimaging, other applications are possible, and the MAGNUS has also been used successfully for limb and prostate imaging.
The first MAGNUS was installed in 2020 at Walter Reed National Military Medical Center in Bethesda, MD, where it is used to study traumatic brain injury in military personnel. Other MAGNUS systems have recently been installed at the University of Wisconsin-Madison and the University of Iowa. The availability of this exceptional system at Brigham and Women’s Hospital will make it accessible to a large community of Boston-area researchers, and we anticipate that it will stimulate many new innovations and discoveries in basic and clinical neuroscience.
The MAGNUS is a head-only insert for a standard GE 3T scanner from which the whole-body gradient coils are removed. The narrow bore diameter at the head allows it to achieve very high gradient performance, comparable to a Siemens Connectom Scanner. This is especially beneficial for diffusion imaging, and it will also be advantageous for other MRI protocols.
The MAGNUS is installed in the BWH MRI Center at 221 Longwood Avenue, Boston. Our center is already equipped with infrastructure to support basic and patient-based research.
The MAGNUS will be available on similar terms to other research scanners through the BWH research imaging core. However, because the MAGNUS is a research-only device, we anticipate that scheduling will be more flexible than for other scanners that are shared with clinical users.
The hourly fee is not yet finalized but will be set in accordance with MGB policy on fee-for-service cores. We expect that it will be comparable to other MRI scanners.
Until the booking system and fee structure is finalized, prospective users are invited to contact the PI, Carl-Fredrik Westin to discuss plans for studies.
As with our current research scanners, the MAGNUS will be open to external as well as internal users.
The MAGNUS is equipped initially with a standard 32-channel head coil, and in future we plan to add a high-performance 64-channel coil that is still under development. There will be an i/v contrast pump to support scans with contrast agents. We also plan to install MR-compatible audio and visual stimulus devices to support task-based fMRI studies. Some of these items are still under discussion, so if you have a specific need or interest, please contact us.
The BWH MAGNUS is an investigational device for research only, and it is not approved for clinical use (although GE has announced future plans to market a clinical version of the MAGNUS, pending FDA approval). The BWH instrument has been installed through a research collaboration between BWH and GE Healthcare. We have an approved IRB protocol under the PI, Carl-Fredrik Westin, to scan research volunteers, and investigators can contact Dr Westin to discuss collaborations. Individual PIs will then need to obtain approval for their specific research projects, as with any imaging study.
The first external system was installed by GE at Walter Reed National Military Medical Center in Bethesda, MD, and has been operational since 2020. MAGNUS scanners have also been installed at University of Iowa and University of Wisconsin-Madison, supported in both cases by NIH instrumentation grants. There is also a testbed MAGNUS system at the GE Research Center in Niskayuna, NY. Another system is scheduled to be installed at King’s College London in 2024, and several other institutions in the US and elsewhere are in discussion with GE about acquiring MAGNUS systems.
Please contact us (see below) and we will be happy to work with you on language and support letters.
If you have specific questions not answered above, please contact any of the following individuals: